没收到验证邮件?请确认邮箱是否正确或 重新发送邮件

#文章仅代表作者观点,不代表IPRdaily立场#
来源:IPRdaily中文网(IPRdaily.cn)
作者:陈钧毅博士 加拿大迪纬律师事务所
原标题:加拿大最高法院否决用“承诺原则”来判定专利实用性 Supreme Court of Canada Rejects “Promise Doctrine” When Determining Patent Utility
2017年6月30日,加拿大最高法院(最高院)公布对阿斯利康加拿大公司诉 Apotex公司一案的判决(阿斯利康案,2017 SCC 36),否决可以用“承诺原则”来判定专利实用性。该判决对如何判定专利是否有实用性,特别是在药学领域,提供了急需的指导。
背景
1、联邦法院建立的专利承诺原则
专利实用性这一要求来自于加拿大《专利法》的第2条 。该法条将发明定义为一种“新的且有用的工艺、流程、机器、制造或构成物”或“新的且有用的”改进。因此,评估实用性时要问的问题是“对什么有用?” 联邦法院的案例以承诺原则来回答这个问题。联邦法院认为如果一个专利承诺了一个特定的用途 (即“专利的承诺”),只有在加拿大申请日时证明了或者合理预测了这个承诺,这个发明才有实用性,但是如果该专利没有承诺任何特定的用途,只要有一点点实用性就可以了。承诺原则要求法院通过考虑权利要求和说明书来确定专利所承诺的实用性。此外,联邦法院认为如果说明书中指定了多项“承诺”,该发明必须满足所有的承诺,否则整个专利将被无效。
自从在2005年左右承诺原则被采纳以来,一批畅销药物的专利被加拿大联邦法院裁定“没有用”。这些药物包括礼来的雷洛昔芬(EVISTA®),阿托莫西汀(STRATTERA®)和奥氮平(ZYPREXA®),赛诺菲-安万特的雷米普利(ALTACE®)和阿斯利康的埃索美拉唑(NEXIUM®)。联邦法院甚至采用了承诺原则裁定欧洲直升机的一项关于直升机起落架的机械专利无效。
2、联邦法院对阿斯利康案的判决
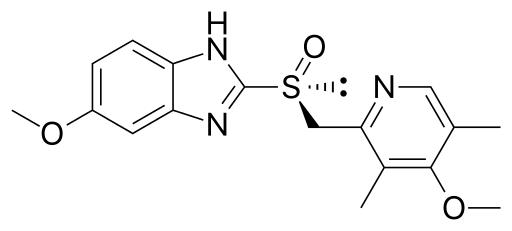
Esomeprazole (埃索美拉唑)
阿斯利康起诉Apotex,称其产品侵权加拿大专利号2139653(653专利),Apotex反诉专利无效。653专利保护奥美拉唑(-)对映体(即埃索美拉唑,见下图)的纯盐和用这些纯盐抑制胃酸分泌。
653专利的说明书有以下阐明:
“人们希望制得具有改善的药代动力学和代谢特性的化合物,这种化合物应能产生改善的治疗特性,例如更低的个体间差异程度。本发明提供了这样的化合物,它们是新的奥美拉唑单一对映体的盐。”[下划线为作者添加]
联邦法院采用了“承诺原则”,并确定了两项承诺的实用性: (1)作为质子抑制剂(PPI),以及 (2)因改进的药代动力学和代谢特性提供了改善的的治疗特性,例如更低的个体间差异程度(2014 FC 638)。联邦法院认为第一项承诺的实用性,即埃索美拉唑作为PPI来降低胃酸量,得到了合理地预测,但是它认为第二项承诺的实用性在加拿大专利申请时既没有被证明也没有被合理预测并因此判定653专利无效。
阿斯利康上诉到联邦上诉法院,但没有成功(2015 FCA 158)。阿斯利康随后请求最高院接受上诉。最高院同意其上诉请求。
最高院阿斯利康案判决
最高院法官一致否决了承诺原则,认为该原则是没有依据的,是与《专利法》的文字和整体规划都不一致的对实用性的解释。最高院认为该原则在以下两个方面过于繁重: (1)“该原则通过专利书中表明的承诺来决定评判实用性的标准”; (2)“如果专利书中表明了多项实用性承诺,该原则要求只有达到所有承诺,专利才能有效”。
特别是最高院认为承诺原则将《专利法》第2条(实用性要求)和第27(3)条(充分描述要求)合并,要求为满足实用性要求,在第27(3)条规定下说明书披露的任何用途都需要在专利申请时被证明或被合理预测。
最高院提出了以下判定专利实用性的方法:
(1)确定专利权利要求所要求的发明的主题;
(2)考虑该主题是否有实用性 - 是否有实际用途(即实际结果)。
最高院指出,这种实用性必须与权利要求所要的发明主题的本质相关,并且只要有一点点实用性就可以了。 因此,只要有一项与发明主题本质相关的实用性就可以了,但是该实用性必须在专利申请日时被证明或被合理地预测。
然而最高院认为过度承诺仍是问题(“mischief”),而且《专利法》对这种行为有多种方式解决:
(1)权利要求范围过宽;
(2)不正确、不完整的披露,或者阐明没有事实根据的用途或者对发明的使用,可能不符合第27(3)条;
(3)过分承诺可能会使专利无效,如果按第53条的规定,过度承诺构成“以误导为目的而故意作出的”疏忽或添加。
在本案中,最高院认为653专利不缺乏实用性,因为联邦法院认为发明的主题 -埃索美拉唑纯盐 - 作为PPI减少胃酸的产生。
简短评论
总而言之,最高院废除承诺原则是对重新统一加拿大与其他主要工业化国家的专利实用性判定的可喜发展。这一案例重申了专利权利要求至上的原则。对于专利申请人和所有者来说,这一判决似乎大大降低了确定专利实用性的门槛。然而,这一判决也表明在加拿大,如果申请人没有在加拿大专利申请时证明或合理预测发明主题的实用性,专利权利要求仍可能受到缺乏实用性的攻击。此外,最高院认为专利权利要求可能因过度承诺而无效,无效理由包括权利要求范围过宽,说明不充分和故意误导的陈述。
附:英文版
Supreme Court of Canada Rejects “Promise Doctrine” When Determining Patent Utility
On June 30, 2017, in AstraZeneca Canada Inc v Apotex Inc (AstraZeneca, 2017 SCC 36), the Supreme Court of Canada (SCC) rejected the “Promise Doctrine” as the correct approach to determine patent utility. This decision provides the much-needed guidance on how to determine patent utility, particularly in the pharmaceutical arts.
Background
1、Development of the Promise Doctrine by the Federal Courts
Section 2 of the Patent Act (Act), the source of the utility requirement, defines an invention as a “new and useful art, process, machine, manufacture or composition of matter” or a “new and useful” improvement thereof. Therefore, the question for assessing utility is “useful for what?”. The Federal Courts’ jurisprudence answered this question with the Promise Doctrine, holding that if a patent promised a specific utility (the “promise of the patent”), only if that promise was fulfilled by either demonstration or sound prediction as of the Canadian filing date of the application, could the invention have the requisite utility. However, where no specific utility was promised, a mere scintilla of utility would suffice. The Promise Doctrine required the courts to identify the promise(s) by considering the claims and the disclosure. Further, if there were multiple “promises” found in the disclosure, the Federal Courts held that the invention must satisfy all of them, or the entire patent would be held invalid.
Since the adoption of the Promise Doctrine in about 2005, a host of blockbuster drug patents were found not to be “useful” by the Canadian Federal Courts. This list included Eli Lilly’s raloxifene (EVISTA®), atomoxetine (STRATTERA®) and olanzapine (ZYPREXA®), Sanofi-Aventis’ ramipril (ALTACE®) and AstraZeneca’s esomeprzole (NEXIUM®). The Federal Courts also applied the Promise Doctrine to invalidate Eurocopter’s mechanical patent for a helicopter landing gear.
2、The Federal Courts’ Decisions in AstraZeneca
AstraZeneca sued Apotex for infringement of its Canadian Patent No. 2,139,653 (653 Patent) and Apotex counterclaimed for a declaration of invalidity. The 653 Patent claims optically pure salts of the (-)enantiomer of omeprazole, esomeprazole (shown below) and use of these salts for inhibiting gastric acid secretion.
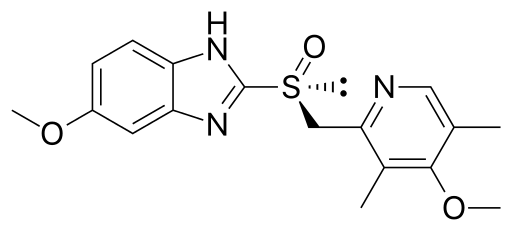
Esomeprazole
The disclosure of the 653 Patents states the following:
“It is desirable to obtain compounds with improved pharmacokinetic and metabolic properties which will give an improved therapeutic profile such as a lower degree of interindividual variation. The present invention provides such compounds, which are novel salts of single enantiomers of omeprazole.” [Underlining added]
The Federal Court (FC) applied the Promise Doctrine and identified two promises of utility: (1) use as a Proton Pump Inhibitor (PPI), and (2) improved pharmacokinetic and metabolic properties which would give an improved therapeutic profile such as a lower degree of interindividual variation (2014 FC 638). The FC found that it was soundly predicted that esomeprazole acted as a PPI to reduce acid in the stomach. However, it found that the second promise was neither demonstrated nor soundly predicted at the Canadian filing date and consequently held that the 653 Patent was invalid.
AstraZeneca appealed to the Federal Court of Appeal (FCA) but was not successful (2015 FCA 158). AstraZeneca then sought leave to appeal to the SCC. The SCC granted the leave.
SCC Decision in AstraZeneca
In an unanimous decision, the SCC rejected the Promise Doctrine, holding that this doctrine is unsound and is an interpretation of the utility requirement that is incongruent with both the words and the scheme of the Act. It held that the doctrine is excessively onerous in two ways: (1) “it determines the standard of utility that is required by reference to the promises expressed in the patent”; and (2) “where there are multiple expressed promises of utility, it requires that all be fulfilled for a patent to be valid”.
In particular, the SCC found that the Promise Doctrine conflates section 2 (utility requirement) and section 27(3) (sufficient description requirement) of the Act by requiring that to satisfy the utility requirement, any disclosed use under section 27(3) be demonstrated or soundly predicted at the time of filing.
The SCC provided the following approach to utility:
(1)identify the subject-matter of the invention as claimed in the patent;
(2)ask whether that subject-matter is useful – is it capable of a practical purpose (an actual result).
The SCC noted that this usefulness must be related to the nature of the claimed subject-matter. It also noted that a scintilla of utility will do. Thus, a single use related to the nature of the subject-matter is sufficient; however, that utility must be either demonstrated or soundly predicted by the filing date.
The SCC nevertheless viewed that overpromising is a “mischief” and that the Act treats this mischief in multiple ways:
(1)claim overbreadth;
(2)a disclosure which is not correct and full, or states an unsubstantiated use or operation of the invention, may fail section 27(3); and
(3)overpromising may void a patent where such statements amount to an omission or addition that is “willfully made for the purpose of misleading,” under section 53.
In this case, the SCC found that the 653 Patent is not invalid for lacking utility, because the FC found the claimed subject-matter – optically pure salts of esomeprazole – to be useful as a PPI to reduce production of gastric acid.
Brief Comments
Overall, the rejection of the Promise Doctrine by the SCC is a favourable development in realigning Canada’s law on patent utility with those of other major industrialized nations. This case reaffirms the primacy of claims in a patent. For patent applicants and owners, this case appears to have significantly lowered the bar to establish patent utility. Nevertheless, this decision also suggests that in Canada, patent claims may still be subject to attacks based on lack of utility if the applicant had not demonstrated or soundly predicted some practical usefulness of the claimed subject-matter by the Canadian filing date. Further, the SCC suggests that patent claims may be held invalid for overpromising on several grounds including overbreadth, insufficiency and wilful misleading statements.
来源:IPRdaily中文网(IPRdaily.cn)
作者:陈钧毅博士,加拿大迪纬律师事务所
编辑:IPRdaily 赵珍 / 校对:IPRdaily 纵横君
推荐阅读

「关于IPRdaily」
IPRdaily成立于2014年,是全球影响力的知识产权媒体+产业服务平台,致力于连接全球知识产权人,用户汇聚了中国、美国、德国、俄罗斯、以色列、澳大利亚、新加坡、日本、韩国等15个国家和地区的高科技公司、成长型科技企业IP高管、研发人员、法务、政府机构、律所、事务所、科研院校等全球近50多万产业用户(国内25万+海外30万);同时拥有近百万条高质量的技术资源+专利资源,通过媒体构建全球知识产权资产信息第一入口。2016年获启赋资本领投和天使汇跟投的Pre-A轮融资。
(英文官网:iprdaily.com 中文官网:iprdaily.cn)

本文来自IPRdaily.cn 中文网并经IPRdaily.cn中文网编辑。转载此文章须经权利人同意,并附上出处与作者信息。文章不代表IPRdaily.cn立场,如若转载,请注明出处:“http://www.iprdaily.cn/”

 共发表文章4693篇
共发表文章4693篇文章不错,犒劳下辛苦的作者吧
- 我也说两句
- 还可以输入140个字













