没收到验证邮件?请确认邮箱是否正确或 重新发送邮件
#本文仅代表作者观点,未经作者许可,禁止转载,不代表IPRdaily立场#
来源:IPRdaily中文网(IPRdaily.cn)
作者:Rachel Bell,Trainee Patent Attorney,麦仕奇英国剑桥办公室
翻译:杨筱杨(Xiaoyang Yang),Associate,麦仕奇北京办公室
关于流感治疗方法和疫苗的已公开专利申请在2018年会达到很高的数目,这也意味着在这一领域的科研和创新在增加。在流感疫苗的专利申请上,中国和美国居于世界领先地位。过去十年中,超过 70%的流感相关优先权申请提交在中国和美国(中国为34.9%,美国为37.6%)。特别值得注意的是,这些申请中,只有大约8%为提交到欧洲专利局的首次申请。
随着深冬到来,最近我们之中肯定有许多人已经因为流感倒下。在多数情况下,流感病程较短,我们很快就能康复。但对于抵抗力弱的人群,比如老年人或那些已经患有基础病的患者,流感则有可能致命。
从2000年起,英国的国民医疗系统(“NHS”)开始提供冬季流感疫苗。然而英国公共卫生部发布的一项关于2017/2018流感季的报告指出,流感导致的死亡人数仍旧令人震惊(去年仅英国就有近16000人死亡)。
在如1918年西班牙大流感的某些极端情况下,流感病毒毒株通过变异可以变得高度致命。尽管西班牙大流感有其时代背景,这场有大约5000万到1亿死亡人数的疫情(相当于全球人口的3%到5%)告诉人们必须研发新的疫苗。
疫苗接种的创新
如同防控任何一种病毒,防控流感的关键在于面对不断演化的病原,所研发的新疫苗和治疗方法能不落其后甚至更为超前,这也就成了演化上的一场猫鼠游戏。在过去几年中,随着高毒性跨物种流感病毒毒株更多地出现,对发现治愈流感的灵药的需求愈发紧迫。
从现有申请递交趋势(见下图)来看,关于流感治疗方法和疫苗的已公开专利申请预计在2018年会达到很高的数目。这也意味着在这一领域的科研和创新在增加。
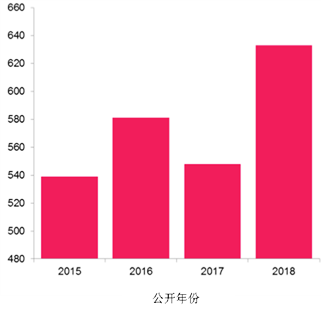
所以,这些申请都是来自哪里,又是由谁提交的呢?我们的分析揭示了一个以激动人心的新科技为发展方向的复杂专利图景。这些新科技能帮助人们从新角度战胜流感。
地理分布—东方vs西方
在流感疫苗的专利申请上,中国和美国居于世界领先地位。过去十年中,超过 70%的流感相关优先权申请提交在中国和美国(中国为34.9%,美国为37.6%)。特别值得注意的是,这些申请中只有大约3%为提交到英国知识产权局的首次申请,8%为提交到欧洲专利局的首次申请。
优先权申请是一项发明在全世界的首次专利申请,因而能很好地反映出特定科技领域的创新程度。在中国提交的大量优先权申请可能反映出创新工作源自中国境内,另一方面美国作为全球重要市场吸收了许多非本国申请。
新疫苗技术
新近的申请中有相当数量是关于现有疫苗的生产改进,如用新技术在疫苗产品中去除内毒素和使用改良的佐剂。此外,新申请中还有一部分是关于对抗流感的新疫苗技术。
例如,最近的一个由Jassen生物科技公司和宾夕法尼亚大学基金会共同提交的申请提到了一种新的AAV(adeno-associated virus,腺相关病毒)疫苗。这项技术的核心在于使用基本上能直接生产出对抗流感病毒的抗体的病毒粒。
这种疫苗使接种疫苗的人无需接触流感病毒即可产生出对抗流感病毒的有效中和抗体。通过AAV载体实现“被动免疫疗法”是一项激动人心的手段,可在今后用于治疗棘手的病毒感染,如流感病毒感染。
MicroRNA治疗
许多关于microRNAs (miRNAs)的申请为诊断和治疗流感提供了可能的新途径。miRNAs是单链RNA结构,作用于人体并能够和信使RNA(mRNA)相互作用,后者在人体中指示蛋白的合成。
中国科学院微生物研究所在此领域提交了很多的申请,在最近的申请中提到了许多和流感有关的miRNAs。微生物研究所发现,这些miRNAs的转基因表达作用于许多重要的病毒mRNA片段,特别是重要的RNA聚合酶亚单元PB2(在流感病毒的转录和复制中起到关键作用的组件),能够限制许多流感病毒的复制。因此,将这些miRNAs给药到病人体内,可以防止mRNA对PB2的指令转化为功能蛋白,从而限制病毒在体内的复制和存活。
在对抗流感的前沿
在防控流感领域多个方向上的创新令人感到欣慰。高毒性流感病毒毒株造成的威胁非常严峻,而城市人口自1918年来的大幅增长(尽管具体数据很难得到,但据世界银行估算,全球城市人口从1960年的33%已增长至现在的55%)又加重了这一威胁的严峻性。在这一背景下,则不难理解防控流感领域中有如此大的创新动力了。
附注:
英国公共卫生部关于2017/2018流感季的报告(英文),见以下链接:https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/740606/Surveillance_of_influenza_and_other_respiratory_viruses_in_the_UK_2017_to_2018.pdf
附:英文全文
FLU VACCINES: ASSESSING THE PATENT AND INNOVATION LANDSCAPE
With winter in full swing, no doubt many of us will have come down with flu recently. On most occasions, influenza is a short-lived illness from which we quickly bounce back. For vulnerable groups however, such as the elderly or those with underlying health conditions, the flu can be deadly.
A winter flu vaccine has been offered on the NHS since 2000. However, a report on the 2017/2018 flu season published by Public Health England indicates that flu still causes a shocking number of deaths (almost 16,000 deaths in the UK alone last year).
In extreme cases also – such as during the so called ‘Spanish flu’ of 1918 – strains of the flu can develop which are far more deadly than most of us experience. While the circumstances surrounding the emergence of Spanish flu were unique to the time, the fact that an estimated 50-100 million people died (somewhere between 3% and 5% of the global population), means new vaccines must be developed.
Innovation in inoculation
As with any virus, containing influenza is a question of developing novel vaccines and therapies that can keep up, or get ahead of, constantly evolving pathogens – a game of evolutionary cat and mouse. With more virulent cross-species flu strains hitting the headlines over the past few years, the pressure to discover a golden bullet treatment – a “cure” for flu – has increased.
As such, the number of published patent applications directed to flu therapeutics and vaccines is projected – based on current filing trends – to reach a notable high in 2018, suggesting that research and innovation in this field has been on the increase.
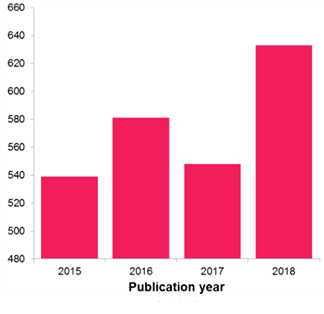
So, where is this filing coming from and who is doing it? Our analysis reveals a complicated patent landscape with a movement towards exciting new technologies that may help defeat flu from new angles.
Geography – East vs West
When it comes to filing patents directed towards flu vaccines, China and the US are leading the world – accounting for more than 70% (34.9% and 37.6%, respectively) of flu-related priority applications filed in the last decade. Notably, only around 3% were first filed at the UK Intellectual Property Office, and 8% at the European Patent Office.
A priority application is a first patent filing of an invention anywhere in the world, and hence tends to be a good indicator of innovation in a particular technology area. Whilst the large volume of priority applications filed in China is likely to reflect innovation coming from China itself, as a major market the US also attracts many non-US applicants.
New vaccine technologies
There have been a significant number of recent applications directed at improving the production of existing vaccines, such as new techniques for removing endotoxins in vaccine products and improved adjuvants. However, in addition, new vaccine technologies against flu have also been demonstrated in recent filings.
For example, a recent filing by joint applicants Janssen Biotech, Inc. and The Trustees of the University of Pennsylvania (US20180243416A1) describes a new AAV (adeno-associated virus) vaccine. The core of this technology is a viral particle which produces, essentially, ready-made antibodies against the flu virus.
One effect of this type of vaccine is that a vaccinated individual is able to produce effective neutralising antibodies against the influenza virus, without the need for exposure to the flu virus. Using AAV vectors for what is known as “passive immunotherapy” is an exciting option for treating challenging viral infections, including influenza, in the future.
MicroRNA treatment
A number of filings directed to microRNAs (miRNAs) offer a new potential avenue for both diagnosis and treatment of flu. miRNAs are single-stranded RNA structures which are processed by the body and capable of interacting with messenger RNA (mRNA) which serve as the instructions for the production of proteins.
For instance, a particularly prolific filer in this area, The Institute of Microbiology, Chinese Academy of Sciences, has recently described a number of miRNAs of relevance in flu. The applicant in this case has found that the transgenic expression of these miRNAs can limit replication of a number of flu viruses by targeting a number of key viral mRNA fragments, in particular PB2 which is an important RNA polymerase subunit – an essential part of flu viral machinery which drives transcription and hence replication of the virus. Delivery of these miRNAs to a patient prevents the conversion of the mRNA instructions for PB2 to a functional protein, therefore limiting the viruses’ ability to replicate and survive in the body.
On the frontier of flu
It is reassuring to see innovation on many fronts in the flu field. Given the seriousness of the threat posed by virulent strains of flu – enhanced by the fact that the urban population has increased significantly since 1918 (data is hard to come by, but the World Bank estimates the global urban population to have increased from 33% in 1960 to 55% at present) – the impetus for innovation is understandable.
Note:
The report on the 2017/2018 flu season published by Public Health England is available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/740606/Surveillance_of_influenza_and_other_respiratory_viruses_in_the_UK_2017_to_2018.pdf
来源:IPRdaily中文网(IPRdaily.cn)
作者:Rachel Bell,Trainee Patent Attorney,麦仕奇英国剑桥办公室
翻译:杨筱杨(Xiaoyang Yang),Associate,麦仕奇北京办公室
编辑:IPRdaily赵珍 校对:IPRdaily纵横君
“投稿”请投邮箱“iprdaily@163.com”

「关于IPRdaily」
IPRdaily成立于2014年,是全球影响力的知识产权媒体+产业服务平台,致力于连接全球知识产权人,用户汇聚了中国、美国、德国、俄罗斯、以色列、澳大利亚、新加坡、日本、韩国等15个国家和地区的高科技公司、成长型科技企业IP高管、研发人员、法务、政府机构、律所、事务所、科研院校等全球近50多万产业用户(国内25万+海外30万);同时拥有近百万条高质量的技术资源+专利资源,通过媒体构建全球知识产权资产信息第一入口。2016年获启赋资本领投和天使汇跟投的Pre-A轮融资。
(英文官网:iprdaily.com 中文官网:iprdaily.cn)
本文来自IPRdaily.cn 中文网并经IPRdaily.cn中文网编辑。转载此文章须经权利人同意,并附上出处与作者信息。文章不代表IPRdaily.cn立场,如若转载,请注明出处:“http://www.iprdaily.cn/”

 共发表文章728篇
共发表文章728篇文章不错,犒劳下辛苦的作者吧
- 我也说两句
- 还可以输入140个字












